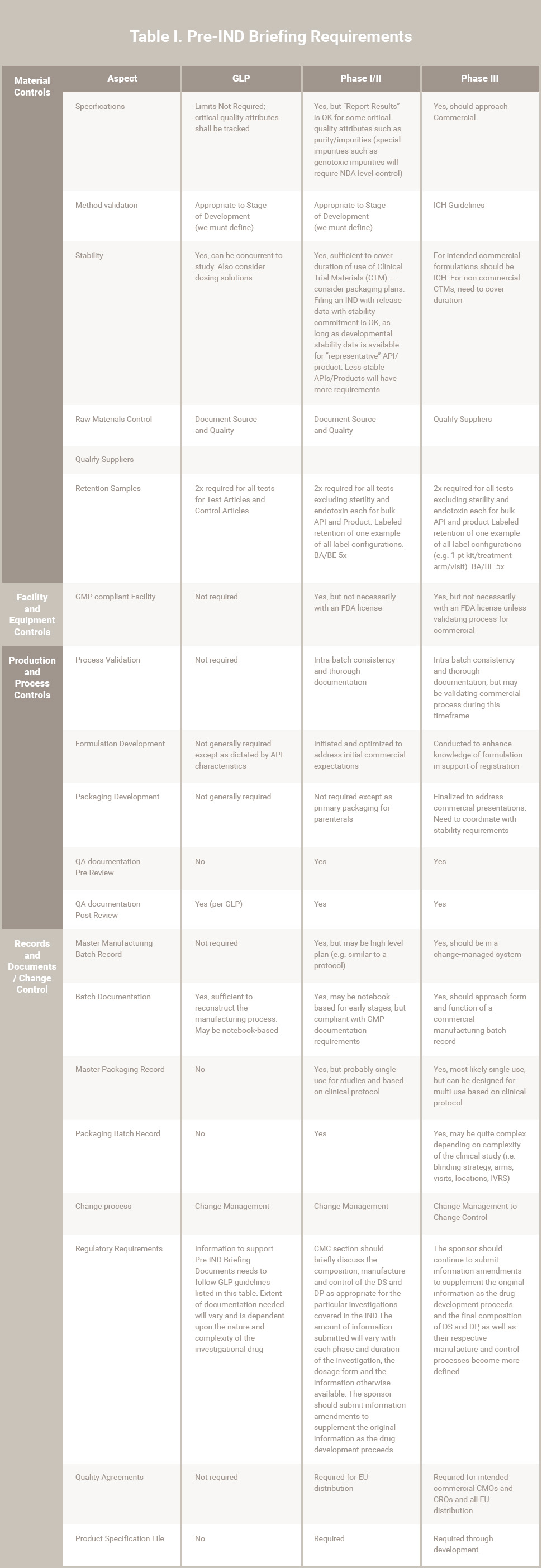
Early Development Gmps For Small-Molecule Specifications . An industry perspective (part i) |. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. Request pdf | on jan 1, 2012, a. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process.
from www.rcainc.com
An industry perspective (part i) |. Request pdf | on jan 1, 2012, a. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development.
GMPs for Early Stage Pharmaceutical Consultants RCA®
Early Development Gmps For Small-Molecule Specifications An industry perspective (part i) |. Request pdf | on jan 1, 2012, a. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. An industry perspective (part i) |.
From www.rcainc.com
GMPs for Early Stage Pharmaceutical Consultants RCA® Early Development Gmps For Small-Molecule Specifications An industry perspective (part i) |. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Request pdf | on jan. Early Development Gmps For Small-Molecule Specifications.
From www.slideserve.com
PPT Best Practices and Application of GMPs for Small Molecule Drugs Early Development Gmps For Small-Molecule Specifications An industry perspective (part i) |. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Request pdf | on jan 1, 2012, a. The development of appropriate control strategies during early development will focus on how. Early Development Gmps For Small-Molecule Specifications.
From www.youtube.com
Difference between GMP (Good Manufacturing Practices)🏭 & GLP (Good Early Development Gmps For Small-Molecule Specifications Request pdf | on jan 1, 2012, a. An industry perspective (part i) |. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Rather than attempting to set specifications/impurity limits based on unoptimized, early. Early Development Gmps For Small-Molecule Specifications.
From www.researchgate.net
Selfrenewal gene signature is enhanced in NHD13/SmoM2 GMPs. a Gene set Early Development Gmps For Small-Molecule Specifications In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. An industry perspective (part i) |. Request pdf | on jan 1, 2012, a. The development of appropriate control strategies during early development will focus on how. Early Development Gmps For Small-Molecule Specifications.
From www.youtube.com
WEBINAR Specifications for Small Molecule Drug Products YouTube Early Development Gmps For Small-Molecule Specifications The development of appropriate control strategies during early development will focus on how phase appropriate specifications. An industry perspective (part i) |. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Request pdf | on jan. Early Development Gmps For Small-Molecule Specifications.
From www.rcainc.com
GMPs for Early Stage Pharmaceutical Consultants RCA® Early Development Gmps For Small-Molecule Specifications In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. An industry perspective (part i) |. Request pdf | on jan 1, 2012, a. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. The development of appropriate control strategies during early development will focus on how. Early Development Gmps For Small-Molecule Specifications.
From www.researchgate.net
CdGMP controls and fluorescence. (A) Different complexes were sampled Early Development Gmps For Small-Molecule Specifications The development of appropriate control strategies during early development will focus on how phase appropriate specifications. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Request pdf | on jan 1, 2012, a. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. An industry perspective. Early Development Gmps For Small-Molecule Specifications.
From slidetodoc.com
Best Practices and Application of GMPs for Small Early Development Gmps For Small-Molecule Specifications Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. Request pdf | on jan 1, 2012, a. An industry perspective. Early Development Gmps For Small-Molecule Specifications.
From www.researchgate.net
Developmental lineage of MDSCs. MMDSCs and GMDSCs arise from a common Early Development Gmps For Small-Molecule Specifications An industry perspective (part i) |. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. Request pdf | on jan 1, 2012, a. The development of appropriate control strategies during early development will focus on how. Early Development Gmps For Small-Molecule Specifications.
From japaneseclass.jp
GMP GMP JapaneseClass.jp Early Development Gmps For Small-Molecule Specifications An industry perspective (part i) |. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. Request pdf | on jan 1, 2012, a. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps. Early Development Gmps For Small-Molecule Specifications.
From www.researchgate.net
The drug development cycle for small molecule drugs and... Download Early Development Gmps For Small-Molecule Specifications An industry perspective (part i) |. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Request pdf | on jan 1, 2012, a. Rather than attempting to set specifications/impurity limits based on unoptimized, early. Early Development Gmps For Small-Molecule Specifications.
From www.slideserve.com
PPT Best Practices and Application of GMPs for Small Molecule Drugs Early Development Gmps For Small-Molecule Specifications The development of appropriate control strategies during early development will focus on how phase appropriate specifications. Request pdf | on jan 1, 2012, a. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. An industry perspective (part i) |. Rather than attempting to set specifications/impurity limits based on unoptimized, early. Early Development Gmps For Small-Molecule Specifications.
From blog.strateos.com
Small Molecule Drug Discovery, HTS and Chemical Libraries Early Development Gmps For Small-Molecule Specifications Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. An industry perspective (part i) |. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Request pdf | on jan. Early Development Gmps For Small-Molecule Specifications.
From en.wikipedia.org
FileGMP chemical structure.png Wikipedia Early Development Gmps For Small-Molecule Specifications In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. An industry perspective (part i) |. Request pdf | on jan. Early Development Gmps For Small-Molecule Specifications.
From www.researchgate.net
CdiGMP metabolism in bacteria. a Synthesis and hydrolysis of Early Development Gmps For Small-Molecule Specifications An industry perspective (part i) |. Request pdf | on jan 1, 2012, a. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Rather than attempting to set specifications/impurity limits based on unoptimized, early. Early Development Gmps For Small-Molecule Specifications.
From www.aging-us.com
The upregulated expression of RFC4 and GMPS mediated by DNA copy number Early Development Gmps For Small-Molecule Specifications Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. An industry perspective (part i) |. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. Request pdf | on jan 1, 2012, a. The development of appropriate control strategies during early development will focus on how. Early Development Gmps For Small-Molecule Specifications.
From www.ptglab.com
GMP Grade Cytokines & Growth Factors Proteintech Group Early Development Gmps For Small-Molecule Specifications Request pdf | on jan 1, 2012, a. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps in early development. The development of appropriate control strategies during early development will focus on how phase appropriate specifications. An industry perspective (part i) |. Rather than attempting to set specifications/impurity limits based on unoptimized, early. Early Development Gmps For Small-Molecule Specifications.
From www.slideserve.com
PPT Specifications Breakout Session 2 Pete Yehl and Mike Coutant Early Development Gmps For Small-Molecule Specifications The development of appropriate control strategies during early development will focus on how phase appropriate specifications. Rather than attempting to set specifications/impurity limits based on unoptimized, early phase ds/dp process. Request pdf | on jan 1, 2012, a. An industry perspective (part i) |. In the june 2012 issue of pharmaceutical technology, a paper written by the iq consortium's gmps. Early Development Gmps For Small-Molecule Specifications.